 MOST PEOPLE would agree that certain events in their life, such as bereavement, changing jobs, examinations, or even rush-hour travel in big cities, are stressful. We try to avoid stress, but if we cannot, we must try to adapt to it. This adaptation is sometimes referred to as ‘toughening up’. Although stress is difficult to define, we know that both avoidance and toughening up are crucial ways of coping with it.
MOST PEOPLE would agree that certain events in their life, such as bereavement, changing jobs, examinations, or even rush-hour travel in big cities, are stressful. We try to avoid stress, but if we cannot, we must try to adapt to it. This adaptation is sometimes referred to as ‘toughening up’. Although stress is difficult to define, we know that both avoidance and toughening up are crucial ways of coping with it.
When we cannot cope, stress can lead to irritability and fatigue, and other more serious disorders, such as gastric ulcers, cardiovascular disease, anxiety and depression. Yet not everyone subjected to severe stress suffers from a heart attack or a bout of depression: some individuals are much more vulnerable than others. Many neuroscientists now suspect that the difference in the ability to cope may lie in biochemical changes in the brain involved in the process of adaptation to stress.
Stress causes the adrenal gland, atop the kidney, to release the hormone adrenaline, but many other hormones, including a related one, noradrenaline, stream into the blood as well. Adrenaline and noradrenaline act in a similar way, causing physiological changes in the body well described by the everyday language used to illustrate stressful experiences, such as ‘hair-raising’, ‘spine chilling’ and ‘cold sweat’. These changes probably prepare us to deal with stress by either ‘flight or fight’, and are remarkably similar to the physiological signs of fear and anxiety. Indeed, drugs that block the actions of adrenaline and noradrenaline can lessen anxiety.
Adrenaline and noradrenaline are also secreted from nerve cells, or neurons, where the compounds act as neurotransmitters, chemical messengers that enable one neuron to communicate with others. Neurons that use noradrenaline as a neurotransmitter feed into nearly all organs in the body, ranging from the iris in the eye, to the gut and the bladder.
The release of such compounds could explain some medical disorders caused by stress. For instance, we know that a high concentration of noradrenaline in the blood, which occurs in people with tumours of the adrenal gland, can fatally damage the muscle of the heart. Stress also increases the levels of this neurotransmitter/hormone in the bloodstream, and could have a similar effect on the heart. The causes of psychiatric disorders, however, are more likely to lie in the brain, so psychopharmacologists have tried to find out how stress alters the way the brain works.
Most studies of the effects of stress on the brain and spinal cord (the central nervous system, or CNS, for short) have looked for changes in the limbic system. This is a collection of regions in the brain involved in the control of emotion and motivation. A network of nerve fibres connects the various parts of the limbic system, linking regions that regulate the secretion of hormones, for instance, to other areas involved in processes such as decision-making and learning.
The neurons of the limbic system rely on many neurotransmitters including adrenaline and noradrenaline. Neurons releasing adrenaline have only recently been discovered and little is known about their function, but we know a bit more about the neurons that release noradrenaline. One popular suggestion is that they serve as an ‘alarm system’, alerting an individual to a conspicuous threat. In support of this idea, researchers have shown that various forms of stress increase the activity of these neurons. The more active the neurons, the more noradrenaline they release.
When the stress is repeated, as frequently happens in everyday life, further changes take place. Neurons produce more molecules of the enzymes needed to synthesise noradrenaline, for instance, enabling the cells to manufacture and release more of this neurotransmitter. Such changes, which show that neurons are adaptable cells, may underlie the ability to cope with stress.
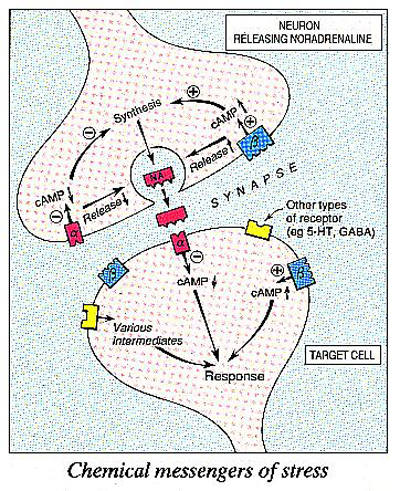
Recently, researchers have tried to find out how stress alters the way neurons in the limbic system communicate. By and large, neurons are not physically connected to each other, but rely on their neurotransmitter to act as a messenger to transmit a nerve impulse from one cell to another. When active, neurons release molecules of their neurotransmitter, which diffuse across the gap, or synapse, separating one neuron from another. To attract the attention of the next neuron in the chain, neurotransmitters in the synapse have to bind to receptors, proteins that lie in the membranes of nerve cells. A popular analogy is that receptors act as biochemical locks which are opened (or closed) by neurotransmitter ‘keys’. Each neurotransmitter has its own specific set of receptors, and each receptor controls a different set of reactions within the target cell. Some receptors, for example, regulate the activity, or firing rate, of target neurons, while others are coupled to enzymes that control the synthesis or release of a neurotransmitter.There are two main types of receptor for noradrenaline, termed a- and b-adrenoceptors.
Noradrenaline activates both types of receptor, but many drugs act on only one receptor type, having no effects on the other. This suggests that there are ‘drug keys’ that will fit the a, but not the b lock, and vice versa. A radioactive label attached to such a drug enables researchers to count the number of receptors for noradrenaline in a sample of tissue, simply by measuring the level of radioactivity in the sample. This technique, known as radioligand binding, has revealed some interesting links between stress and receptors for noradrenaline.
The first is that stress affects the number of both a- and b- adrenoceptors. A single exposure to stress produces unpredictable changes in adrenoceptors which probably depend on a range of factors, such as the duration and severity of the stress and an animal’s prior experience of that particular form of stress. When the stress is repeated, more consistent changes appear. In particular, the number of b-adrenoceptors in the brain falls. This happens in untamed rats even after stress as mild as that of simply handling them for a minute a day.
The most remarkable aspect of this change is that it seems to require several daily sessions. The process of ‘taming’, judging from outward signs of ‘handling stress’ such as tremor and attempts to bite any fingers that stray too near to the ‘dental defence system’, also takes several days of repeated handling. A reduction in the number of receptors for noradrenaline could underlie this process of taming. That is, the changes in receptors might be a component of adaptation to stress.
We now know how this might happen. When noradrenaline binds to the b-adrenoceptors of a neuron, the cell produces more cyclic AMP, an important compound that regulates a wide range of biochemical reactions inside cells, including the synthesis and release of neurotransmitters. After repeated stress, however, noradrenaline is less able to stimulate the cell to produce cyclic AMP. By binding to b-adrenoceptors and modifying the production of cyclic AMP, noradrenaline has a ‘hot line’ into biochemical processes regulating the function of target neurons. So stress not only increases the release of noradrenaline, but also affects target cells by making their b-adrenoceptors less sensitive to noradrenaline.
The ability of repeated stress to reduce the synthesis of cyclic AMP is especially interesting because studies with radioligand binding show a similar change in the brain cells of animals given antidepressant drugs for several days. This finding has been an important milestone in research on antidepressant drugs. Eric Stone, working at the State University of New York in the US, has suggested that antidepressant drugs can help to reduce depression precisely because they produce changes in b-adrenoceptors that mimic the adaptation to stress. It follows from this that depression may be caused by a failure of the mechanisms responsible for adaptation to stress. The theory could account for the link between stress and depression, and for the fact that some individuals are particularly vulnerable to stress.
At this point, we must confront another big problem: what does noradrenaline actually do in the brain? Is the increased release of this neurotransmitter responsible for the unpleasant effects of stress, or does it help us to overcome them? The answer to this question is not clear and scientific opinion is deeply divided.
One school of thought has it that the release of noradrenaline in the brain is directly responsible for the harmful emotional effects of stress. This conclusion is based mainly on evidence collected from experiments on animals where researchers electrically stimulated neurons releasing noradrenaline. This procedure causes changes in emotions and behaviour that strongly resemble anxiety and the response to stress. According to this theory, a reduction in the number of b-adrenoceptors after repeated stress helps to counteract the harmful effects of noradrenaline.
If noradrenaline causes the bad effects of stress, we should be able to abolish these effects by preventing the release of noradrenaline. There are a number of ways of doing this, but the simplest is to use a drug such as 6-hydroxydopamine which, when injected into the brain, selectively destroys the neurons that use noradrenaline as a neurotransmitter. When Philippe Soubrie, working at the National Institute of Health and Medical Research (INSERM) in Paris, did this he found that animals were still vulnerable to stress. Other studies have found that injections of noradrenaline into certain regions of the brain could prevent the animals from developing the symptoms of stress. Findings such as these suggest that the release of noradrenaline itself is not responsible for the harmful effects of stress but is a physiological response designed to overcome them. So the release of noradrenaline may be crucial for the process of ‘toughening up’.
Even antidepressant drugs do not work in brains bereft of noradrenaline. Researchers have known for some time that removing noradrenaline from the brain prevents antidepressant drugs from reducing the number of b-adrenoceptors. So evidence is growing that noradrenaline helps to reduce the consequence of stress, probably by reducing the number of b-adrenoceptors. Noradrenaline is certainly not the only factor regulating the number of b-adrenoceptors, however. Other hormones are also probably involved in this process. These include the glucocorticoids (also known as stress hormones) which, like adrenaline and noradrenaline, are produced by the adrenal gland.
Other neurotransmitters within the limbic system also seem to influence the number of -adrenoceptors in the brain. Two of these, 5-hydroxytryptamine (or 5-HT, for short) and -aminobutyric acid (or GABA, for short), may be particularly important. If 5-HT is removed from the brain, antidepressants no longer reduce the number of -adrenoceptors, perhaps because the receptors become less responsive to noradrenaline. No one yet knows how the removal of 5-HT changes the way receptors respond to stress, but this could provide another important clue to the link between stress, anxiety and depression. The drug company Bristol-Myers has recently introduced a drug that seems to reduce the release of 5-HT, as a new form of treatment for anxiety.
Like 5-HT, GABA may also influence the number of adrenoceptors. Drugs that enhance the actions of GABA, a neurotransmitter that inhibits neurons that carry receptors for it, have long been known for their effectiveness in treating anxiety. They include Valium and Librium, which belong to a group of compounds known as benzodiazepines (see ‘Anything for a quiet life?’, New Scientist, 6 May 1989). There is some recent evidence to suggest that drugs that block GABA – so-called inverse agonists of benzodiazepines – increase the number of b-adrenoceptors in the brain. People taking an inverse agonist in trials said they felt extremely anxious, and they showed physiological changes like those seen in stressed people. So these drugs may mimic the effects of stress.
Studies such as these suggest that all the changes in neurotransmitters and their receptors caused by stress may be interrelated. Thus, drugs acting on one group of neurons may have far-reaching effects in the brain. Indeed, this could explain why both antianxiety and antidepressant drugs seem to relieve anxiety or depression whether they modify the function of noradrenaline, 5-HT or GABA. If this is the case, an imbalance of the function of different neurotransmitters, rather than a disorder of any one in particular, could cause stress-related disorders. Only when we understand all these processes will we be able to explain why some people cannot cope with stress, while others can.


Very quickly this web site will be famous among all
blogging users, due to it’s pleasant posts